Cardiac Anatomy and Physiology
Introduction
The heart is a remarkable organ that serves as the engine of the circulatory system, pumping blood to deliver oxygen and nutrients throughout the body. Understanding the heart’s anatomy and physiology is essential for accurately interpreting echocardiographic images, as each structure and function provides critical insights into cardiovascular health.
This section explores the heart’s chambers, valves, coronary arteries, and electrical conduction system, along with the cardiac cycle and pressure-volume relationships. By connecting these foundational concepts with echocardiographic imaging, you’ll gain the tools to assess cardiac function and diagnose abnormalities effectively.
Left ventricle
The left ventricle (LV) is a powerful, elliptical-shaped chamber designed to pump oxygen-rich blood into systemic circulation. Its geometry, resembling a prolate spheroid, supports efficient contraction and high-pressure output needed to sustain the body’s demands.
Functionally, the LV pumps blood into the aorta during systole, achieving an ejection fraction (EF) of 55–70% under normal conditions. It also relaxes during diastole to fill from the left atrium without excessive pressure. Both phases are essential for maintaining cardiac output and detecting dysfunctions like heart failure.
In summary, the LV’s unique geometry, robust walls, and precise segmentation allow it to efficiently sustain systemic circulation, making it central to cardiac health and echocardiographic assessment.
Right ventricle
The right ventricle (RV) is a crescent-shaped chamber that pumps deoxygenated blood to the lungs. Its thinner walls (3–5 mm) and lower pressure system (15–30 mmHg systolic) are suited for pulmonary circulation. The RV’s geometry is more complex than the left ventricle, with three regions: the inlet (tricuspid valve), body (main chamber), and outflow tract (leading to the pulmonary artery).
Functionally, the RV contracts sequentially—longitudinally and radially—sufficient for low-pressure pulmonary flow. Echocardiography evaluates RV size, wall thickness, and motion, with the free wall being key for assessing function. Pathologies like pulmonary hypertension or RV infarction can impair its performance, highlighting its importance in heart health.
Left atrium
The left atrium (LA) is a thin-walled chamber that collects oxygenated blood from the lungs via the pulmonary veins and channels it into the left ventricle. Positioned posteriorly, the LA acts as a reservoir during ventricular systole, a conduit during early diastole, and a booster pump during atrial systole, contributing 20–30% of ventricular filling. Its size and function are critical for maintaining efficient blood flow, and enlargement often reflects pressure overload from conditions like hypertension or mitral valve disease. The left atrial appendage is a key site for thrombus formation, particularly in atrial fibrillation, increasing stroke risk. Echocardiography assesses LA size and volume, which are essential for evaluating diastolic function and heart failure.
Right atrium
The right atrium (RA) is a thin-walled chamber located anterosuperiorly to the left atrium, receiving deoxygenated blood from the superior and inferior vena cavae and directing it into the right ventricle. It functions as a reservoir during ventricular systole, a conduit during early diastole, and a booster pump during atrial systole, contributing significantly to ventricular filling. Key structures include the right atrial appendage, the fossa ovalis (a remnant of fetal circulation), the crista terminalis (a ridge separating the smooth posterior wall from the trabeculated anterior wall), and the Chiari network (a mobile, web-like structure near the inferior vena cava). Echocardiography assesses RA size and function, which are critical for evaluating right heart pressures and detecting abnormalities like thrombi, with enlargement often linked to tricuspid regurgitation, pulmonary hypertension, or right-sided heart failure.
Mitral valve
The mitral valve is a bicuspid valve situated between the left atrium (LA) and left ventricle (LV), regulating unidirectional blood flow while withstanding high systemic pressures. It consists of two leaflets: the larger, mobile anterior leaflet and the smaller, scalloped posterior leaflet. These are anchored to the heart by the annulus, a fibrous ring, and tethered to the papillary muscles in the LV via chordae tendineae, which prevent prolapse during systole. During diastole, the valve opens to allow blood flow from the LA to the LV, while during systole, it closes tightly to prevent backflow. Proper function relies on the coordination of its components, including the zones of coaptation, where the leaflets meet and seal. Dysfunction, such as stenosis, regurgitation, or prolapse, can significantly impair cardiac output and lead to clinical symptoms. The mitral valve’s structure and function are vital for efficient heart performance and are key areas of focus in echocardiography.
Click the ‘play button’ on the right hand side for an excellent lecture and in-depth analysis of mitral valve anatomy and physiology from the The Cardiothoracic Surgery Network.
Aortic valve
The aortic valve is a semilunar valve located between the left ventricle (LV) and the aorta, regulating blood flow from the heart into the systemic circulation. It is structured to withstand high pressures and ensure unidirectional blood flow.
Anatomically, the aortic valve has three cusps: the left coronary cusp, right coronary cusp, and non-coronary cusp. These crescent-shaped cusps are anchored to the aortic annulus, a fibrous ring that provides structural support. The valve opens passively during systole when ventricular pressure exceeds aortic pressure, allowing oxygenated blood to flow into the aorta. During diastole, the cusps close tightly to prevent backflow into the LV, ensuring efficient circulation and maintaining coronary artery perfusion as the valve’s closure creates pressure for coronary flow.
The sinuses of Valsalva, small pouches behind each cusp, play a critical role in valve mechanics by reducing cusp stress and facilitating coronary artery blood flow. The aortic valve’s robust structure and precise function are essential for maintaining efficient systemic circulation.
Variations in the valve structure are clinically significant. A bicuspid aortic valve, where two cusps fuse during development, is the most common congenital abnormality, often leading to stenosis or regurgitation over time. Rarer variations include unicuspid or quadricuspid valves, which may also cause functional impairment and require monitoring.
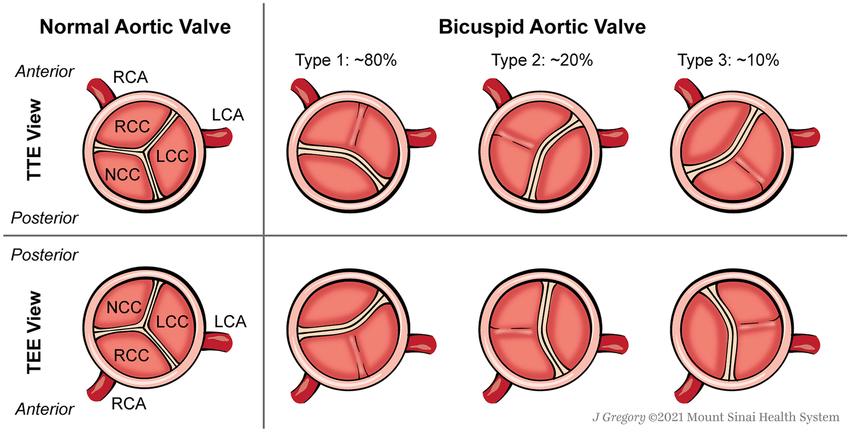
Tricuspid valve
The tricuspid valve is an atrioventricular valve located between the right atrium (RA) and right ventricle (RV), facilitating unidirectional blood flow. It is composed of three leaflets: the anterior, posterior, and septal leaflets, which are supported by the surrounding fibrous annulus. The anterior leaflet is the largest and most prominent, the posterior leaflet is smaller, and the septal leaflet is attached to the interventricular septum.
The leaflets are tethered by chordae tendineae, which connect to the papillary muscles of the RV. This arrangement prevents the valve leaflets from prolapsing into the RA during ventricular contraction. The tricuspid valve opens during diastole, allowing deoxygenated blood to flow from the RA into the RV, and closes during systole to prevent backflow. As part of the low-pressure pulmonary circulation, the tricuspid valve operates at lower pressures compared to the left-sided valves, ensuring efficient filling and emptying of the right ventricle.
Pulmonary valve
The pulmonary valve is a semilunar valve located between the right ventricle (RV) and the pulmonary artery, ensuring unidirectional blood flow from the heart to the lungs. It consists of three cusps: the anterior, right, and left cusps, which are anchored to the pulmonary annulus, a fibrous ring that provides structural support.
During systole, the pulmonary valve opens passively as the RV contracts, allowing deoxygenated blood to flow into the pulmonary artery. During diastole, the valve closes as the pressure in the pulmonary artery exceeds that in the RV, preventing backflow of blood into the heart. The cusps form a tight seal during closure, ensuring efficient forward blood flow. The pulmonary valve operates under lower pressure compared to the aortic valve, reflecting its role in the low-resistance pulmonary circulation. Its precise structure and function are crucial for maintaining effective blood flow to the lungs for oxygenation.
Cardiac physiology
This section explores the fundamental processes that drive heart function, including the cardiac cycle, where we examine the intricate sequence of systole and diastole. We’ll delve into pressure and volume relationships, highlighting the Frank-Starling mechanism and its role in optimizing cardiac output. Additionally, we’ll uncover the heart’s electrical conduction system, the foundation of coordinated contractions and rhythm. Together, these topics provide a comprehensive understanding of how the heart efficiently pumps blood to sustain life.
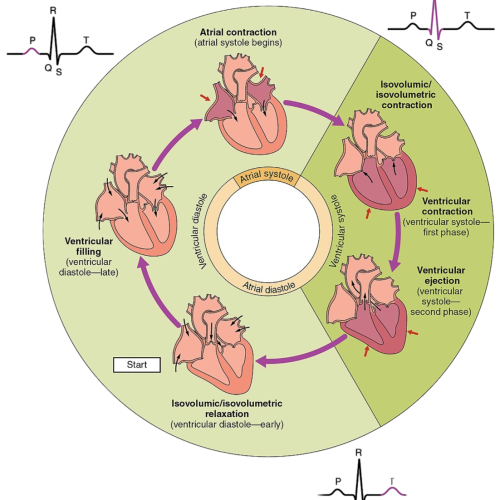
The cardiac cycle (as seen above)
Diastole (Relaxation Phase): The cardiac cycle begins with diastole, where the heart relaxes to allow for ventricular filling. Isovolumetric Relaxation: During this initial phase of diastole, the ventricles relax after systole, and the aortic and pulmonary valves close to prevent backflow of blood. At this point, all valves are closed, and no blood enters the ventricles. Ventricular pressure drops rapidly, preparing the heart for the next phase of filling. Ventricular Filling: Once the pressure in the ventricles falls below atrial pressure, the mitral (left side) and tricuspid (right side) valves open. Blood flows passively from the atria into the ventricles, accounting for the majority of ventricular volume. This process is driven by pressure differences and occurs without active contraction. Atrial Systole: During late diastole, the atria contract to push additional blood into the ventricles, contributing approximately 20–30% of ventricular filling. This “atrial kick” ensures optimal preload for the ventricles and is especially important when ventricular compliance is reduced, such as in certain pathological conditions.
Systole (Contraction Phase): Systole begins with the contraction of the ventricles, leading to blood ejection into the systemic and pulmonary circulations. Isovolumetric Contraction: At the start of systole, the ventricles contract while all valves remain closed. This phase generates pressure in the ventricles but does not involve a change in volume. The buildup of pressure is critical for opening the aortic and pulmonary valves during the next phase. Ventricular Ejection: Once the ventricular pressure exceeds the pressure in the aorta and pulmonary artery, the aortic and pulmonary valves open. Blood is ejected from the left ventricle into the systemic circulation and from the right ventricle into the pulmonary circulation. This phase continues until the ventricles begin to relax, at which point the valves close to prevent backflow.
Pressure and volume relationship
The heart’s ability to adapt to changes in pressure and volume is essential for maintaining efficient blood flow. These relationships govern how the heart fills during diastole and ejects blood during systole, ensuring optimal cardiac output under varying physiological demands.
Frank-starling mechanism
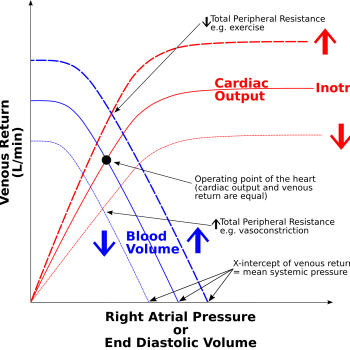
The Frank-Starling principle states that the heart’s stroke volume increases in response to greater end-diastolic volume (preload). As the ventricles fill with more blood, the myocardial fibres stretch, allowing the heart to contract with greater force during systole. This relationship ensures that cardiac output matches venous return, maintaining efficient circulation.
Key Points:
- Mechanism: Increased sarcomere length enhances actin-myosin overlap and calcium sensitivity, boosting contractility.
- Physiological Role: Adapts cardiac output to changes in venous return, such as during exercise or fluid shifts.
- Limitations: Excessive stretching (e.g., in heart failure) impairs contraction, reducing efficiency.
Clinical relevance
In heart failure, the heart’s inability to pump blood effectively leads to an increase in end-diastolic volume (EDV). This occurs because reduced ejection fraction leaves more residual blood in the ventricles after each contraction. Over time, venous return adds to this residual blood, further increasing the volume.
To compensate, the body activates the Frank-Starling mechanism, where increased preload temporarily enhances stroke volume. However, chronic volume overload overstretches myocardial fibres, leading to inefficient contraction and ventricular dilation. Additionally, neurohormonal responses, like activation of the renin-angiotensin-aldosterone system (RAAS), promote fluid retention and vasoconstriction, increasing blood volume and venous return.
This volume overload contributes to congestion, with blood backing up into the lungs in left-sided failure or into systemic circulation in right-sided failure, causing pulmonary edema or peripheral swelling. While initially compensatory, these mechanisms ultimately worsen heart failure symptoms.
LaPlace’s Law
Laplace’s Law describes the relationship between wall stress, pressure, radius, and wall thickness in a hollow structure like the heart. It is mathematically expressed as:
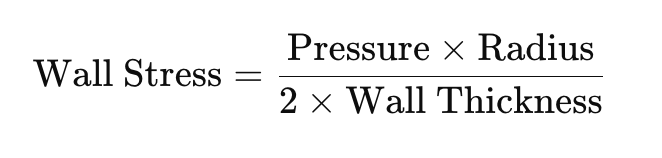
What it means for the heart
- Wall Stress: Wall stress is the tension the myocardial fibres experience to generate pressure and maintain structural integrity.
- Pressure: As pressure within the ventricle increases (e.g., during systole), wall stress rises proportionally.
- Radius: An increase in ventricular size or radius (e.g., in dilated cardiomyopathy) significantly increases wall stress because stress is proportional to the radius.
- Wall Thickness: Increased wall thickness reduces wall stress, as thicker walls distribute the pressure more evenly. This explains ventricular hypertrophy as a compensatory mechanism in pressure-overloaded hearts (e.g., in hypertension or aortic stenosis).
Clinical relevance
- In conditions like dilated cardiomyopathy, the ventricle enlarges (increased radius), leading to higher wall stress and inefficient contraction, which worsens cardiac function.
- In pressure overload conditions like chronic hypertension or aortic stenosis, the heart adapts by developing concentric hypertrophy (increased wall thickness) to reduce wall stress.
- High wall stress also increases myocardial oxygen demand, contributing to ischemia in certain conditions.


